Liquid crystals behave differently than normal crystalline compounds. While most compounds undergo change from solid to liquid phase at the melting point, Liquid crystals transition from solid phase to liquid phase via one or more intermediate phases (mesophases) designated as nematic, smectic or cholesteric.
Frinton Laboratories, Inc. supplies a number of liquid crystals which may be divided into 4 categories:
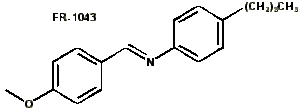
- Schiff bases, of general structure PhCH=NPh with various substituents mostly in the para position. These were the earliest liquid crystals discovered. they have very low transition temperatures, and some exhibit nematic properties at room temperature. The most common is N-(4-methoxybenzylidene)-4-butylaniline (MBBA) (Frinton no. 1043) which exhibits a room temperature nematic phase.
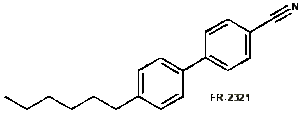
2. Cyanobiphenyls, of general structure RPh-PhCN, where R is an alkyl chain and both substituents are in the 4-position. their transition temperatures are a bit higher than those of the schiff bases. These liquid crystals have become very useful in digital displays and other uses. Frinton Laboratories, Inc. supplies a large number cyanobiphenyls and terphenyls. An important example is 4-cyano-4’-hexylbiphenyl (6CB) (FR-2321) which also exhibits a room temperature mesophase.
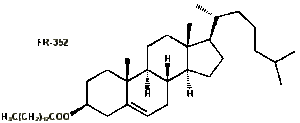
3. Cholesteryl  esters: these exhibit a cholesteric phase which is caused by the chirality of the cholesterol moiety. An example is cholesteryl tetradecanoate (or myristate) (FR-0352).
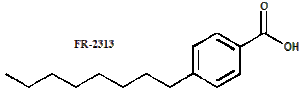
 4. Alkyl- and  alkoxy-benzoic acids, of general structure RPhCOOH, where R is an alkyl or alkoxy group para to the carboxy. Examples are 4-octylbenzoic acid (FR-2313) and 4-octtyloxybenzoic acid (FR-0799).
4. Alkyl- and  alkoxy-benzoic acids, of general structure RPhCOOH, where R is an alkyl or alkoxy group para to the carboxy. Examples are 4-octylbenzoic acid (FR-2313) and 4-octtyloxybenzoic acid (FR-0799).
Get in Touch
For any inquiries or assistance, feel free to reach out to our knowledgeable team:
- Toll-Free Call in the USA: 1-877-FRINTON (1-877-374-6866)
- Direct Contact: 856-722-7037
- Fax: 856-439-1977
We look forward to providing you with the support you need.

